FOR PHYSICIANS
SEARCH
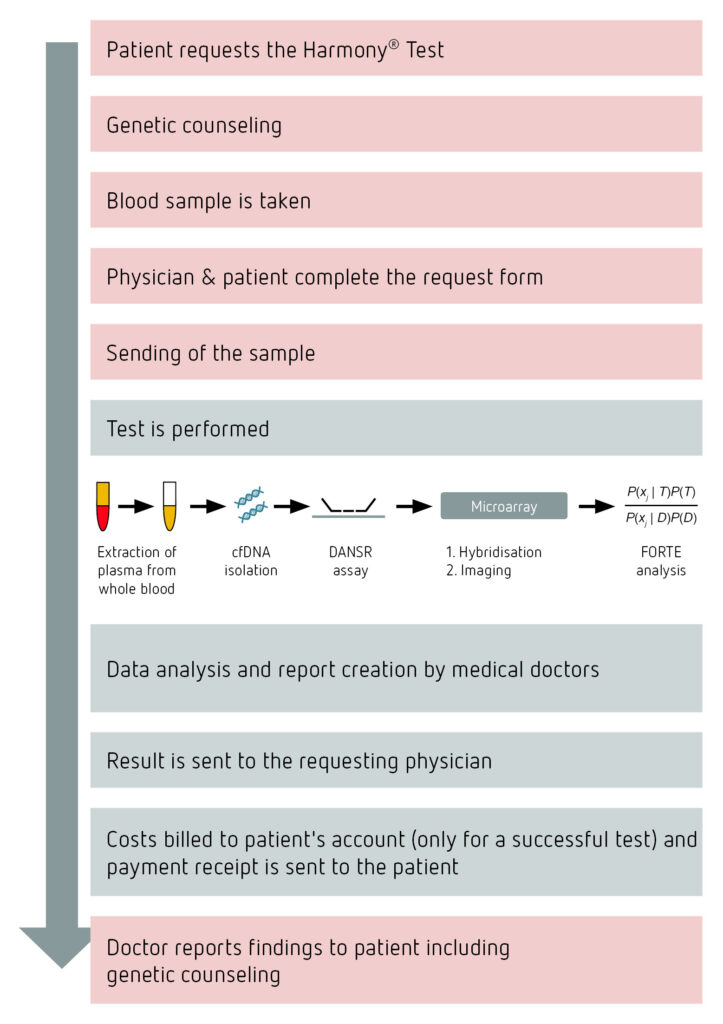
Harmony® Test procedure
The Harmony® Test can be performed from week 10+0 of the pregnancy. Before carrying out a Harmony® Test, the patient should be given an ultrasound scan to determine the gestational age and to check whether it is a singleton or multiple pregnancy.
The Harmony® Test can be performed with all singleton or twin pregnancies, irrespective of the manner of conception (including in vitro fertilisation and egg donation). Data indicates that the detection rate for twin pregnancies (93%) is slightly lower than for singleton pregnancies.1,2 It is not yet possible to test twin pregnancies for sex chromosome anomalies (X, Y) such as Turner or Klinefelter syndromes and the DiGeorge syndrome.
The table below provides an overview of which Harmony® Test options are possible with different types of pregnancies.
| Harmony® Test | ||||
| Harmony® with with X/Y analysis | ||||
| DiGeorge syndrome | ||||
| sex determination |
An important requirement for the performance of the Harmony® Test is detailed, open and objective patient counseling in accordance with the German Genetic Diagnostics Act. If the patient decides to take the test, 2 x 9 ml of venous blood should be drawn into special blood tubes (provided). After the request form has been fully completed, the two blood tubes should be packed as described in the instructions (included in the sampling kit) and sent by post or courier.
The cell-free fetal DNA remains stable for approximately 9 days in the special test tubes. Cenata will only accept samples that are not more than 7 days old. The samples should thus be sent as quickly as possible after the blood draw. The samples should stay at room temperature and never be cooled or frozen since haemolysis will occur, and the test cannot be analysed.
The test is evaluated using CE-certified software.
On average it takes 3 working days from its receipt to process the sample until the results are returned. This means that you will usually have the results for your patient on the 5th working day after the blood sample was taken.
If the fetal sex is to be determined during the test, the patient may only be informed of this after week 12+0 of the pregnancy in accordance with the Genetic Diagnostics Act (post-conception, corresponding to week 14 of the pregnancy when calculated starting after the last period). If the patient is not yet in week 14+0 of the pregnancy when the results are ready we will send you (the requesting physician) partial findings that do not contain information on the sex of the fetus. When week 14+0 of the pregnancy is reached we will automatically send you the report on the sex of the fetus.
Each patient sample undergoes a multi-stage quality control process in our laboratory.
The first quality control takes place directly at the sample entrance. Here it is determined whether the durability, the duration of transport, the information on the request form as well as the quantity and consistency of the sample meet the specified quality requirements.
During processing in the laboratory, each analysis must pass a variety of quality control criteria. These are listed in the overview below:
| Parameter | Description |
| Array Quality | General microarray quality |
| Fetal Fraction | Amount of cell-free fetal DNA relative to maternal cell-free DNA |
| Signal | Signal intensity of the measurement |
| DNA Quality | Indication of the overall quality of the analysis. Indication of another DNA species in the sample. |
| Noise | Intensity of the background signal |
| Signal to Noise | Indication of the ratio of the signal intensity to the background signal |
Only when all parameters reach a minimum value, an analysis result is recieved, and the detailed report is created.
This is followed by the third stage of result release – medical validation. Each report is reviewed and approved by a team of qualified physicians and scientists.
In a small number of cases, the proportion of fetal DNA (cffDNA) in the blood may be too low for results to be obtained. In these cases, Cenata will automatically repeat the test. If no clear results are obtained from the repeat test, the report will be returned as “not analysable” and Cenata will not charge the patient for the test. A new blood sample may be submitted at a slightly later date, as the amount of fetal DNA (Fetal Fraction) in the mother’s blood increases with the length of the pregnancy.
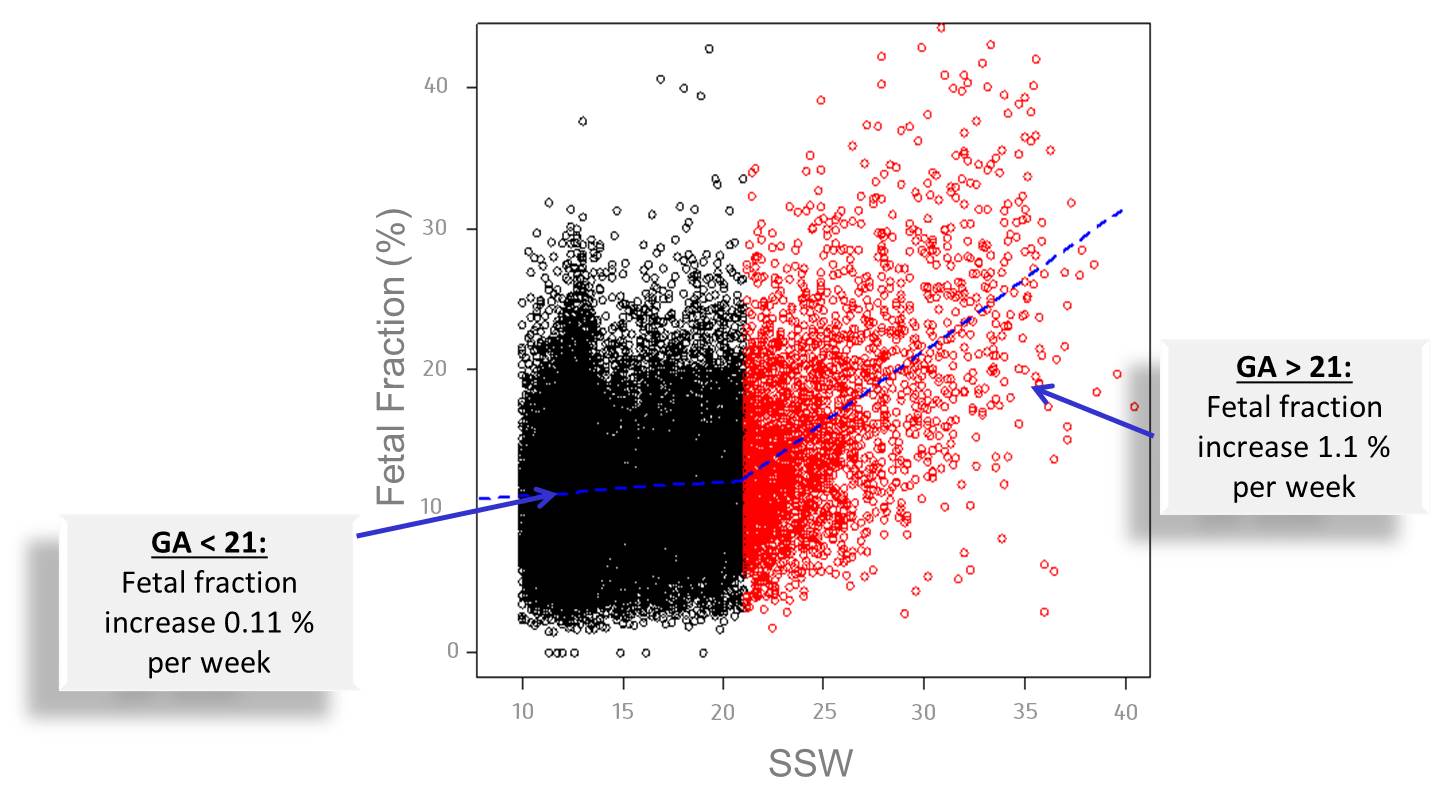 Figure 1: Relationship of the “Fetal Fraction” and the week of pregnancy (SSW) (modified acc. to 3)
Figure 1: Relationship of the “Fetal Fraction” and the week of pregnancy (SSW) (modified acc. to 3)
Other reasons why the test may fail include the existence of an egg donation about which we were not informed, a “vanishing twin” situation, or previously unknown maternal chromosomal disorders.
If you need help interpreting the findings or have any questions on the methods used in the Harmony® Test, you can consult our team of qualified physicians including specialists in human genetics, laboratory medicine and obstetrics/gynaecology.
Free kits with the CE-labelled blood collection tubes for the blood samples and the transport material can be ordered in the following ways:
- using our contact form
- by fax: +49 7071 565 44 444
- by phone +49 7071 565 44 430
The order request form to be completed by the patient is included with the sampling kit and can also be downloaded from our homepage.
- Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015 Jan;45(1):61-6. doi: 10.1002/uog.14690. Epub 2014 Dec 4. ↩
- del Mar Gil M, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204-11. doi: 10.1159/000356495. Epub 2013 Nov 15. ↩
- Wang E1, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013 Jul;33(7):662-6. doi: 10.1002/pd.4119. Epub 2013 May 9. ↩
FOR PHYSICIANS
SEARCH
Harmony® Test procedure
The Harmony® Test can be performed from week 10+0 of the pregnancy. Before carrying out a Harmony® Test, the patient should be given an ultrasound scan to determine the gestational age and to check whether it is a singleton or multiple pregnancy.

The Harmony® Test can be performed with all singleton or twin pregnancies, irrespective of the manner of conception (including in vitro fertilisation and egg donation). Data indicates that the detection rate for twin pregnancies (93%) is slightly lower than for singleton pregnancies.1,2 It is not yet possible to test twin pregnancies for sex chromosome anomalies (X, Y) such as Turner or Klinefelter syndromes and the DiGeorge syndrome.
The table below provides an overview of which Harmony® Test options are possible with different types of pregnancies.
| Harmony® Test | ||||
| Harmony® with with X/Y analysis | ||||
| DiGeorge syndrome | ||||
| sex determination |
An important requirement for the performance of the Harmony® Test is detailed, open and objective patient counseling in accordance with the German Genetic Diagnostics Act. If the patient decides to take the test, 2 x 9 ml of venous blood should be drawn into special blood tubes (provided). After the request form has been fully completed, the two blood tubes should be packed as described in the instructions (included in the sampling kit) and sent by post or courier.
The cell-free fetal DNA remains stable for approximately 9 days in the special test tubes. Cenata will only accept samples that are not more than 7 days old. The samples should thus be sent as quickly as possible after the blood draw. The samples should stay at room temperature and never be cooled or frozen since haemolysis will occur, and the test cannot be analysed.
The test is evaluated using CE-certified software.
On average it takes 3 working days from its receipt to process the sample until the results are returned. This means that you will usually have the results for your patient on the 5th working day after the blood sample was taken.
If the fetal sex is to be determined during the test, the patient may only be informed of this after week 12+0 of the pregnancy in accordance with the Genetic Diagnostics Act (post-conception, corresponding to week 14 of the pregnancy when calculated starting after the last period). If the patient is not yet in week 14+0 of the pregnancy when the results are ready we will send you (the requesting physician) partial findings that do not contain information on the sex of the fetus. When week 14+0 of the pregnancy is reached we will automatically send you the report on the sex of the fetus.
Each patient sample undergoes a multi-stage quality control process in our laboratory.
The first quality control takes place directly at the sample entrance. Here it is determined whether the durability, the duration of transport, the information on the request form as well as the quantity and consistency of the sample meet the specified quality requirements.
During processing in the laboratory, each analysis must pass a variety of quality control criteria. These are listed in the overview below:
| Parameter | Description |
| Array Quality | General microarray quality |
| Fetal Fraction | Amount of cell-free fetal DNA relative to maternal cell-free DNA |
| Signal | Signal intensity of the measurement |
| DNA Quality | Indication of the overall quality of the analysis. Indication of another DNA species in the sample. |
| Noise | Intensity of the background signal |
| Signal to Noise | Indication of the ratio of the signal intensity to the background signal |
Only when all parameters reach a minimum value, an analysis result is recieved, and the detailed report is created.
This is followed by the third stage of result release – medical validation. Each report is reviewed and approved by a team of qualified physicians and scientists.
In a small number of cases, the proportion of fetal DNA (cffDNA) in the blood may be too low for results to be obtained. In these cases, Cenata will automatically repeat the test. If no clear results are obtained from the repeat test, the report will be returned as “not analysable” and Cenata will not charge the patient for the test. A new blood sample may be submitted at a slightly later date, as the amount of fetal DNA (Fetal Fraction) in the mother’s blood increases with the length of the pregnancy.
 Figure 1: Relationship of the “Fetal Fraction” and the week of pregnancy (SSW) (modified acc. to 3)
Figure 1: Relationship of the “Fetal Fraction” and the week of pregnancy (SSW) (modified acc. to 3)
Other reasons why the test may fail include the existence of an egg donation about which we were not informed, a “vanishing twin” situation, or previously unknown maternal chromosomal disorders.
If you need help interpreting the findings or have any questions on the methods used in the Harmony® Test, you can consult our team of qualified physicians including specialists in human genetics, laboratory medicine and obstetrics/gynaecology.
Free kits with the CE-labelled blood collection tubes for the blood samples and the transport material can be ordered in the following ways:
- using our contact form
- by fax: +49 7071 565 44 444
- by phone +49 7071 565 44 430
The order request form to be completed by the patient is included with the sampling kit and can also be downloaded from our homepage.
- Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015 Jan;45(1):61-6. doi: 10.1002/uog.14690. Epub 2014 Dec 4. ↩
- del Mar Gil M, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204-11. doi: 10.1159/000356495. Epub 2013 Nov 15. ↩
- Wang E1, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013 Jul;33(7):662-6. doi: 10.1002/pd.4119. Epub 2013 May 9. ↩
Harmony® Test procedure
The Harmony® Test can be performed from week 10+0 of the pregnancy. Before carrying out a Harmony® Test, the patient should be given an ultrasound scan to determine the gestational age and to check whether it is a singleton or multiple pregnancy.

The Harmony® Test can be performed with all singleton or twin pregnancies, irrespective of the manner of conception (including in vitro fertilisation and egg donation). Data indicates that the detection rate for twin pregnancies (93%) is slightly lower than for singleton pregnancies.1,2 It is not yet possible to test twin pregnancies for sex chromosome anomalies (X, Y) such as Turner or Klinefelter syndromes and the DiGeorge syndrome.
The table below provides an overview of which Harmony® Test options are possible with different types of pregnancies.
| Harmony® Test | ||||
| Harmony® with with X/Y analysis | ||||
| DiGeorge syndrome | ||||
| sex determination |
An important requirement for the performance of the Harmony® Test is detailed, open and objective patient counseling in accordance with the German Genetic Diagnostics Act. If the patient decides to take the test, 2 x 9 ml of venous blood should be drawn into special blood tubes (provided). After the request form has been fully completed, the two blood tubes should be packed as described in the instructions (included in the sampling kit) and sent by post or courier.
The cell-free fetal DNA remains stable for approximately 9 days in the special test tubes. Cenata will only accept samples that are not more than 7 days old. The samples should thus be sent as quickly as possible after the blood draw. The samples should stay at room temperature and never be cooled or frozen since haemolysis will occur, and the test cannot be analysed.
The test is evaluated using CE-certified software.
On average it takes 3 working days from its receipt to process the sample until the results are returned. This means that you will usually have the results for your patient on the 5th working day after the blood sample was taken.
If the fetal sex is to be determined during the test, the patient may only be informed of this after week 12+0 of the pregnancy in accordance with the Genetic Diagnostics Act (post-conception, corresponding to week 14 of the pregnancy when calculated starting after the last period). If the patient is not yet in week 14+0 of the pregnancy when the results are ready we will send you (the requesting physician) partial findings that do not contain information on the sex of the fetus. When week 14+0 of the pregnancy is reached we will automatically send you the report on the sex of the fetus.
Each patient sample undergoes a multi-stage quality control process in our laboratory.
The first quality control takes place directly at the sample entrance. Here it is determined whether the durability, the duration of transport, the information on the request form as well as the quantity and consistency of the sample meet the specified quality requirements.
During processing in the laboratory, each analysis must pass a variety of quality control criteria. These are listed in the overview below:
| Parameter | Description |
| Array Quality | General microarray quality |
| Fetal Fraction | Amount of cell-free fetal DNA relative to maternal cell-free DNA |
| Signal | Signal intensity of the measurement |
| DNA Quality | Indication of the overall quality of the analysis. Indication of another DNA species in the sample. |
| Noise | Intensity of the background signal |
| Signal to Noise | Indication of the ratio of the signal intensity to the background signal |
Only when all parameters reach a minimum value, an analysis result is recieved, and the detailed report is created.
This is followed by the third stage of result release – medical validation. Each report is reviewed and approved by a team of qualified physicians and scientists.
In a small number of cases, the proportion of fetal DNA (cffDNA) in the blood may be too low for results to be obtained. In these cases, Cenata will automatically repeat the test. If no clear results are obtained from the repeat test, the report will be returned as “not analysable” and Cenata will not charge the patient for the test. A new blood sample may be submitted at a slightly later date, as the amount of fetal DNA (Fetal Fraction) in the mother’s blood increases with the length of the pregnancy.

Other reasons why the test may fail include the existence of an egg donation about which we were not informed, a “vanishing twin” situation, or previously unknown maternal chromosomal disorders.
If you need help interpreting the findings or have any questions on the methods used in the Harmony® Test, you can consult our team of qualified physicians including specialists in human genetics, laboratory medicine and obstetrics/gynaecology.
Free kits with the CE-labelled blood collection tubes for the blood samples and the transport material can be ordered in the following ways:
- using our contact form
- by fax: +49 7071 565 44 444
- by phone +49 7071 565 44 430
The order request form to be completed by the patient is included with the sampling kit and can also be downloaded from our homepage.
- Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015 Jan;45(1):61-6. doi: 10.1002/uog.14690. Epub 2014 Dec 4. ↩
- del Mar Gil M, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204-11. doi: 10.1159/000356495. Epub 2013 Nov 15. ↩
- Wang E1, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013 Jul;33(7):662-6. doi: 10.1002/pd.4119. Epub 2013 May 9. ↩


